Curiosities and Challenges about Physics-Chemistry
Do you like Physics-Chemistry?
Would you like to see more curiosities like these..
Carbon Dioxide (CO2) can solidify when cooled below -78 °C, in this state it is known as dry ice. When heated to....
When we cut onions, two cells present in them are released: one rich in sulphides and the other in enzymes. When these..
To neutralize the effects of a bottle of Coke, you need to drink 32 glasses of water. This drink contains about 10....
So.. How about a Challenge on the Best Quiz Platform in the World?
Every scene is an opportunity to shine
Get to know a little about everything and bet on your knowledge in incredible challenges and duels..
Join the betspot.zone community, accumulate bts (bets) and even compete for prizes..
Discover some interesting facts about Physics-Chemistry..
academic Quiz - academic Curiosities -academic Challenge - school Quiz - school Curiosities - school Challenge - physics Quiz - physics Curiosities - physics Challenge - chemistry Quiz - chemistry Curiosities - chemistry Challenge - - Frequently asked questions about Physics-Chemistry
Heat is a form of energy that

Heat is a form of energy that is transferred from one body to another when there is a difference in temperature between them, resulting in thermal equilibrium. The standard unit of measurement for heat is the Joule (J), although it is common to use calories (cal) for measurement. Heat and temperature are distinct concepts, with the first being energy and the second a measure of the agitation of particles in a body. Throughout history, the concept of heat has been debated by philosophers and scientists, from Aristotle to Lavoisier and Lord Kelvin, who established that heat is a form of energy. Heat can be transferred by conduction, convection and radiation, the latter being a propagation of electromagnetic waves that does not require a physical medium to occur, following the Steffan-Boltzmann Law, where the amount of heat emitted is proportional to the fourth power of the temperature of the body (Q α T^4).
publicity
The art of pyrotechnics, dating back millennia

The art of pyrotechnics, dating back millennia, had its origins in ancient China, where natives threw pieces of green bamboo into bonfires during festivities to scare away evil spirits with loud sounds. Later, Chinese alchemists created gunpowder by mixing saltpeter, sulfur and charcoal, initially looking for an elixir of eternal life, but ended up using it in fireworks. This practice spread across Europe and the United States, becoming an integral part of celebrations. Originally orange, the fireworks acquired vibrant colors after the addition of metals by Italian inventors in 1830. The colors of the fireworks are determined by the compounds used, such as barium chloride for green, magnesium for silver, lithium for red and sodium for yellow.
Organic chemistry
Organic chemistry is the branch of chemistry that studies organic compounds, which contain carbon in their molecular structure. These compounds are present in nature and in synthetic products manufactured by humans. Organic chemistry focuses on the structure, properties, synthesis and reactivity of organic compounds. Structural formulas and functional groups are used to represent and identify the compounds. Topics covered include nomenclature, structure and properties, organic synthesis, reaction mechanisms and reactivity of functional groups. Organic chemistry is essential in many industrial sectors such as pharmaceuticals, polymers, food, energy and general chemicals. Its development contributes to society and science.
Stoichiometric calculations

Stoichiometric calculations are used in chemistry to determine amounts of substances in a chemical reaction. The basic steps include: Write the balanced chemical equation for the reaction. Identify the known amounts of substances involved in the reaction. Convert known amounts to moles, if necessary. Use the stoichiometric ratio of the balanced equation to perform ratio calculations. Calculate the unknown amount of the desired substance. It is important to consider the limiting reagent, which determines the maximum amount of product formed in the reaction. Stoichiometric calculations are useful for determining the amount of reactants needed, predicting the amount of products formed, and solving problems related to quantities in chemical reactions.
publicity
Chemical radioactivity

Chemical radioactivity is a phenomenon in which unstable atoms emit subatomic particles or electromagnetic radiation to achieve greater nuclear stability. There are three main types of radiation: alpha particles, beta particles and gamma radiation.Alpha (α) particles: These are helium nuclei composed of two protons and two neutrons. They have a positive charge and limited penetration. Beta particles (β): These are high energy electrons or positrons. They have a lower charge and mass than alpha particles and have a greater penetration capacity. Gamma radiation (γ): It is a form of electromagnetic radiation without charge and mass. It is highly energetic and has the greatest penetrating ability. Radioactivity occurs in unstable atoms known as radioactive isotopes, which are transformed into more stable isotopes through radioactive decay. Alpha decay (α), Beta decay (β) and Gamma radiation emission (γ).
Molar mass
Molar mass is a physical property that indicates the mass of a substance on a molecular scale. It is calculated by adding up the atomic masses of all the atoms present in a molecule or chemical formula. The unit of measurement for molar mass is grams per mole (g/mol). This unit indicates how many grams of a substance are present in 1 mole of that substance. For example, if the molar mass of a substance is 32 g/mol, that means that 1 mol of that substance weighs 32 grams. To calculate the molar mass of a substance, you need to know the atomic masses of the elements that make it up. The atomic mass of an element is found on the periodic table of elements. It represents the weighted average of the masses of the isotopes of that element.
Carbon Dioxide (CO2) can solidify when cooled below -78 °C

Carbon Dioxide (CO2) can solidify when cooled below -78 °C, in this state it is known as dry ice. When heated to atmospheric pressure, it passes directly to the gaseous state, without passing through the liquid (sublimation). For CO2 to become liquid, a pressure greater than 5 atmospheres is required. When warm air is blown over dry ice, a dense white cloud forms at ground level, an effect often used in theaters. Its sublimation temperature is 194.7 K (-78.5 °C; -109.2 °F) at Earth's atmospheric pressure. However, its use must be done with caution, as direct contact with the extremely cold solid can cause frostbite. While generally not toxic, if released indoors it can lead to hypercapnia (abnormally high levels of carbon dioxide in the blood).
publicity
When we cut onions

When we cut onions, two cells present in them are released: one rich in sulphides and the other in enzymes. When these cells mix, a reaction occurs that produces an acid called sulfenic acid. This acid is transformed into gas and, when in contact with water, generates a weak solution of sulfuric acid. As the eyes are always wet, the solution formed causes irritation in the eyes, which causes tears. That's why we cry when we cut onions.
To neutralize the effects of a bottle of Coke

To neutralize the effects of a bottle of Coke, you need to drink 32 glasses of water. This drink contains about 10 teaspoons of sugar, which is 100% of the recommended daily intake. Phosphoric acid controls the taste of sugar. Ten to twenty minutes after eating, there is an increase in insulin production due to the spike in blood sugar. After 40 minutes, caffeine is fully absorbed, increasing blood pressure, dilating pupils and blocking adenosine receptors, causing insomnia. Five minutes later, dopamine stimulates the pleasure centers in the brain. After 60 minutes, the acids present in Coca-Cola react with nutrients, such as magnesium, zinc and calcium, in the large intestine, being eliminated in the urine, accompanied by water and sodium. Finally, enthusiasm gives way to tiredness and irritation due to lack of sugar.
When inhaling helium
When inhaling helium, the speed of propagation of the voice increases, resulting in a thinner sound. This is due to the lightness of the gas, which is approximately 7 times lighter than air made up of 78% nitrogen (N2). On the other hand, inhaling a denser gas would cause the voice to propagate at a lower speed, which would make it more serious.
publicity
After consuming alcoholic beverages
After consuming alcoholic beverages, alcohol is almost completely absorbed by the digestive tract and enters the bloodstream. On an empty stomach, absorption may take 15 to 20 minutes, and may vary depending on the alcohol concentration, drink composition, simultaneous intake of food and milk, among other factors. Alcohol is produced by a fermentation process from fruits, honey, stems, cereals such as barley, grapes and sugar cane. Alcoholic beverages can be fermented or distilled, with distilled beverages being more graduated. Alcohol is a psychoactive substance that has depressant effects on the central nervous system, affecting the behavior of people who consume it. This substance also interferes with cognitive and perceptive abilities, especially in vision and hearing.
Naphthalene is a hydrocarbon that
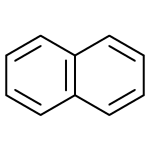
Naphthalene is a hydrocarbon that is part of the aromatic group. It is used to prevent the destruction of natural fibers, such as wool and cotton, through its ability to sublimate, that is, to change from a solid to a gaseous state, releasing vapors that kill larvae and moths. For this to happen safely, it is necessary that the substance is stored in sealed packages. However, when someone is directly exposed to naphthalene vapors or wears clothing that has been exposed to it, there is a risk of developing adverse health effects such as hemolytic anemia, restlessness, diarrhea, blood in the urine, and cataracts in the eyes with prolonged exposure.
Isoprene is a special diene, very present in our lives
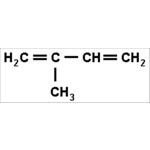
Isoprene is a special diene, very present in our lives. Its molecular formula is C5H8. When we link two or more units of this molecule, we form open or closed chains called Terpenes. These Terpenes, or their oxygenated derivatives, are the main components of essential oils, colored substances and rubber products. Natural rubber, or latex, is found in the sap of some trees, such as the rubber tree, and its formula repeats the units of isoprene in the order of 5000. This natural rubber formation reaction results in the production of numerous products made from rubber. , such as condoms, surgical gloves, balloons, school erasers, among others.
publicity
Acetylsalicylic acid, better known as aspirin
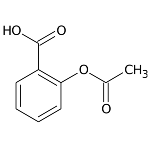
Acetylsalicylic acid, better known as aspirin, is a mixed-function organic compound that offers excellent anti-rheumatic, anti-febrile and analgesic properties. In 1829, pharmacist H. Leroux was the first to isolate salicin from the bark of the willow plant. Later, in 1859, the German chemist Kolbe synthesized salicylic acid in his laboratory. However, this compound had unpleasant effects, such as stomach irritation. It was then that, in 1897, the pharmacist Felix Hoffman obtained acetylsalicylic acid, by carrying out a reaction between salicylic acid and acetic anhydride. Since then, this compound has been used to treat headaches, rheumatic fever and other ailments.
Acrylamide, also known as Pop-2-enamide

Acrylamide, also known as Pop-2-enamide, is an organic molecule from the functional group of amides. It has three carbon atoms and a pi bond between two of them. Commonly found in industrialized foods, it is not added to products, but originates from the chemical reaction between asparagine (enzyme) and glucose (carbohydrate) when they are heated to a temperature equal to or greater than 120°C. It is also present in cigarette smoke. In addition, Acrylamide has applications in civil construction, as it can be polymerized in the form of polyacrylamide.
Aniline
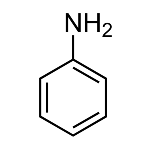
Aniline is an aromatic benzeneamine substance that was first isolated in 1826 with the destructive distillation of indigo. It received this name because the plant from which it was obtained was called Indigofera anil. It is usually used commercially because it is cheap and can be used in the manufacture of different types of dyes. When prepared, it appears as a colorless, oily liquid that darkens easily when exposed to light. Furthermore, it is toxic and has an odor similar to rotten fish, as it belongs to the amine group. For these characteristics, her name is used as a synonym for dye.
publicity
Since 1847, trichloromethane, better known as chloroform

Since 1847, trichloromethane, better known as chloroform, has been used for medicinal purposes as an anesthetic. However, due to its high toxicity, this organic compound has been replaced by other substances. Additionally, chloroform was used for recreational purposes, such as at parties, where people inhaled the substance to numb themselves. Nowadays, the illegal use of drugs such as "loló" and "perfume lance", which are composed predominantly of chloroform and ethyl ether, is still common. The consumption of these products can cause euphoria, hallucinations and dizziness, but also depression, mental confusion, paleness, convulsions and even coma.
In 1868

In 1868, Pierre-Jules-César Janssen noticed a yellow spectral line on the Sun that was unlike hydrogen and any other known element on Earth. The English astronomer Norman Lockyer gave the name helium to this element, which remained undiscovered for 25 years here on Earth. In 1895, William Ramsay treated gases produced by cleveite with acids and sent them to William Crookes and Lockyer, who realized that it was helium gas. In Switzerland, Per Cleve and his student Nils Abraham Langlet also discovered the same, thus being considered discoverers of helium. Helium is the second lightest and most abundant element in the Universe, after hydrogen. It is widely used mainly to inflate balloons, especially at children's parties.
Nitroglycerin

Nitroglycerin was discovered in 1847 by the Italian chemist Ascanio Sobrero when he reacted glycerin with a sulphonitric mixture. It was very unstable and its explosion was extremely violent, which made it impossible to use it as an explosive. In 1867, Swedish chemist Alfred Bernhard Nobel managed to make nitroglycerin less sensitive to shocks by creating dynamite, a mixture of 75% nitroglycerin and 25% diatomaceous earth. Later, Nobel also invented gelatinous dynamite and smokeless gunpowder. Currently, nitroglycerin has applications in medicine as a coronary vasodilator, being prescribed in cases of risk of infarction and obstruction of the arteries.
publicity
Diamonds are an allotropic form of carbon

Diamonds are an allotropic form of carbon, with each carbon atom bonded to four others, forming a structure that can only form in the deepest layers of the Earth, due to high pressures and temperatures. These are then expelled into the earth's crust, where synthetic diamonds are also created by modifying the crystalline structure of graphite. The different colors of diamonds can be caused by impurities such as nitrogen for the yellow color or boron for the blue color. Another cause could be deformations of the crystalline lattice, resulting in rare brown, pink or red diamonds.
If placed in the fridge can the battery work again? Well

If placed in the fridge can the battery work again? Well, the overall reaction in the battery consists of the transfer of electrons from zinc to the cathode, which is composed of manganese dioxide, powdered charcoal, and a slurry of ammonium chloride and zinc chloride. When this reaction takes place, the dioxide is converted to manganese trioxide, which is irreversible. The battery will only work as long as electrons continue to be transferred to the cathode. However, throughout the process, ammonia (NH3) is released, depositing itself on the graphite bar, making it difficult for electrons to pass through and causing the battery to discharge. Placing the battery in the fridge will not revert it from this state and therefore will not get it working again.
Thermochemistry or Chemical Thermodynamics

Thermochemistry or Chemical Thermodynamics is the branch of chemistry that studies the behavior of chemical systems in relation to energy. It involves analyzing the energy changes associated with chemical reactions, as well as the equations of state that describe these relationships. Chemical Thermodynamics also addresses the thermodynamic properties of substances, such as enthalpy, latent heat, Gibbs free energy, entropy, and others, which determine the chemical equilibrium of a system. These properties can be used to predict the outcome of chemical reactions and to calculate the energy potential of chemical systems.
publicity
Optical phenomena happen when light and matter interact
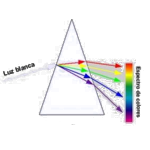
Optical phenomena happen when light and matter interact. Reflection, refraction, absorption, scattering and interference of light are the main factors responsible for these events. Reflection makes it possible for us to see objects around us, and refraction occurs when light changes speed as it passes through transparent media. Absorption happens due to certain pigments. Nature is full of optical events such as Northern Lights, Mirage, Crepuscular Rays, Anti-Crepuscular Rays, Solar Halo, Rainbow, Pillars of Light, Iridescent Clouds and Belt of Venus. These are proofs of the interaction of light with matter, being true examples of optical wonders.
Sound
Sound is a longitudinal and mechanical wave that propagates in material media such as air, water, metal, etc. It is produced by vibrations capable of creating areas of compression and rarefaction in atmospheric gases that alternate according to the frequency of the source. The speed of propagation of sound waves is determined by the elasticity of the medium, being greater the more elastic it is. Sound has properties such as speed, wavelength, frequency and amplitude. The Doppler effect, in turn, is the increase or decrease in the apparent frequency of a sound due to the relative motion between a sound source and an observer. We can calculate the speed of sound if we know its frequency and wavelength.
Specific heat

Specific heat is an intensive physical quantity that describes the thermal variation of a substance when it receives a certain amount of heat. Also known as mass heat capacity, it is measured in units such as J/(kg.K) or cal/(g °C). Specific heat is calculated using the formula c = Q/m. ΔT or c = C/m. In addition, the molar specific heat, also called the molar heat capacity, is calculated by the relationship between the heat capacity and the number of moles present. Latent heat, in turn, is the amount of heat received or given off by a body, where its temperature remains the same, while its physical state changes. This quantity is measured by the calculation C = Q/ΔT.
publicity
Atoms are made up of three fundamental particles: Protons
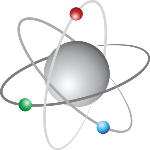
Atoms are made up of three fundamental particles: Protons, Neutrons and Electrons. The nucleus accounts for most of the atomic mass and the electron cloud determines the size of the atom. Atoms can acquire or lose electrons, forming ions with an electrical charge, such as anions (which gain electrons) and cations (which lose electrons). The mass of the atom is concentrated in its nucleus, as the mass of protons and neutrons is the same. Isotopes are atoms of the same chemical element, so they have the same atomic number but different mass number due to the fact that they have different numbers of neutrons.
The periodic table
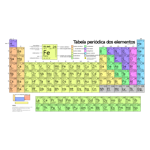
The periodic table is a system of classifying chemical elements by ordering them by atomic numbers, electron configurations, and periodic patterns of properties. This organization shows recurring trends, such as similar elements in the same columns. There are also four rectangular blocks that have similar chemical properties. As a rule, in the same line (period) the elements on the left are metallic and those on the right are non-metallic. These rows are called periods, while the columns are called groups. In addition to numbering, each group has a specific name, such as halogens (group 17) and noble gases (group 18). This table is used to understand relationships between element properties and to predict the characteristics of elements not yet discovered or produced. It provides a useful basis for understanding chemical behavior and is used extensively in chemistry and other areas of knowledge.
The Gravitational Force
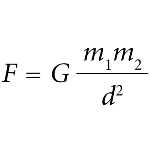
The Gravitational Force is the mutual interaction between two bodies that is attractive and never repulsive. It is what allows us to stand up, as the Earth exerts gravitational force on bodies. This force makes the Earth's translational movement happen, as well as the other planets. The Law of Universal Gravitation, proposed by Isaac Newton, explains the height of the tides, the life cycle of stars and the maintenance of living stars. The weight of a body is the result of the action of the Earth' gravity field on it. The attractive force of gravity is equal to the weight, neglecting the rotation of the Earth and the action of gravity exerted by the Sun, Moon and other stars. The acceleration due to gravity g is approximately 9.83 m/s² near the Earth's surface.
publicity
A magnetic field
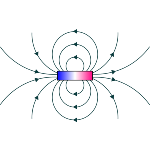
A magnetic field is a region of space that exerts forces on moving electrical charges or materials with magnetic properties. This physical quantity is measured in tesla (T) and is generated through the movement of electrically charged particles, as well as the alignment of magnetic domains inside the magnets. According to the laws of electromagnetism, these magnetic fields originate from the variation of the electric field. Since the way in which the magnetic domains are organized influences the type of magnetism present in the material, materials can be classified as ferromagnetic, diamagnetic or paramagnetic, depending on the strength with which they are attracted or repelled by an external magnetic field.
pH
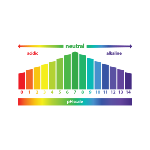
pH is a measure of the acidity or alkalinity of an aqueous solution, with values ranging from 0 to 14. 0 is the most acidic and 14 is the most alkaline, while 7 is neutral, having no effect on the system. The acronym "pH" stands for "hydrogen potential" and is used to measure the ratio of H3O+ to OH- ions in solution. According to the Arrhenius acid-base theory, acids are substances that release hydronium ions (H3O+) into water, and bases are substances that release hydroxyl ions (OH-). Water undergoes the autoionization process, which means that it contains the same amount of H3O+ and OH- in its composition, defining the pH of the solution.
Back